First Medical Trial: A Fetus Receives a Life-Saving Drug in the Womb
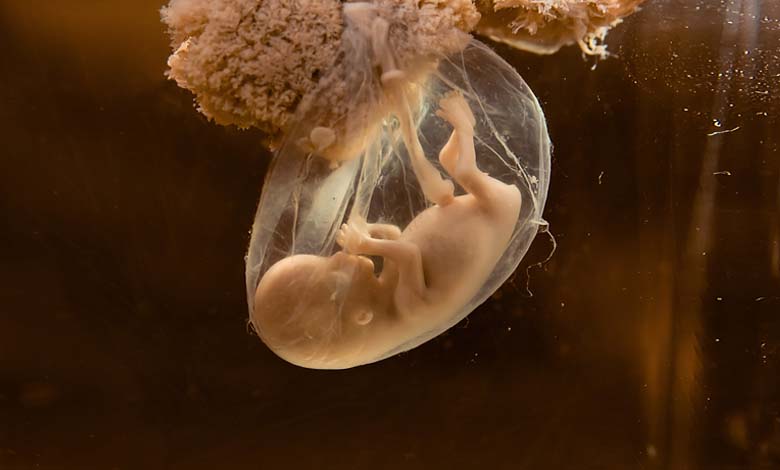
Doctors in the United States have, for the first time, successfully treated a fetus suffering from a congenital disease that develops rapidly in the womb.
According to Science Alert, the fetus had severe neuromuscular complications, prompting the mother to agree to administer the drug while still pregnant.
-
50 Million Views: A Rare Birth of Twins in Two Separate Uteruses
-
Why are young people more prone to cancer?
A prenatal test revealed that the fetus carried two genetic mutations indicative of Type 1 Spinal Muscular Atrophy (SMA), a condition that usually leads to severe muscle weakness and breathing difficulties within six months of birth.
Historically, most children with Type 1 Spinal Muscular Atrophy do not survive beyond their second birthday, usually due to respiratory failure.
However, the child in this study has now reached the age of two and a half without showing any symptoms.
-
First in Jordan: Successful Kidney Transplant without Matching Blood Types of Patient and Donor
-
Development of a test to detect brain cancer… The First and Fastest in the World
The child’s parents had previously lost a child to spinal muscular atrophy. When tests confirmed that their unborn baby also had this progressive neuromuscular disease, they sought to find out if treatment could begin earlier.
For this particular case, the U.S. Food and Drug Administration (FDA) approved the early administration of risdiplam, an oral medication commercially known as Evrysdi, owned by the pharmaceutical company F. Hoffmann-La Roche AG.
Over the years, randomized clinical trials have demonstrated that risdiplam is safe and effective in treating spinal muscular atrophy in newborns. The younger the children are when they begin receiving the drug, the better the overall outcomes.
-
“A Serious Mistake”: Two Children Consume Birth Control Pills in Turkey
-
Does Artificial Intelligence Provide Accurate Health Advice?
In this new study, the pregnant mother took a daily dose of risdiplam for six weeks before birth. Tests showed that the drug passed directly through the umbilical cord blood and the amniotic fluid surrounding the fetus. After birth, the infant received the medication orally every day, rather than the mother.
Pediatric neurologist Michelle Farrar, who specializes in minimally invasive gene therapy for spinal muscular atrophy in Australia, stated in Nature that the baby treated for SMA “was effectively cured, showing no signs of the condition” even after 30 months of life.
-
The Oddities of Leaders: “Titans” Defeated by Phobias
-
Turkey: Scissors Forgotten in a Patient’s Abdomen for 7 Years
In 2020, the FDA had first approved risdiplam for children with spinal muscular atrophy, but only for those older than two months.
However, recent clinical trials funded by Roche found that most children treated with risdiplam before the age of six weeks were able to swallow, feed, sit, stand, and walk independently after two years of treatment. None of them required permanent ventilatory support.












